(music: Gary McFarland's 1969 cover of The Beatles' Get Back)
Here is the relevant part of the abstract from Dr Yu et al's paper (the full text is still behind a paywall):
After adjustments for age, sex, race, presence of cirrhosis, body mass index, treatment with peginterferon, lifetime alcohol consumption, smoking, health status, and coffee and macronutrient intake, each higher quartile of cholesterol intake was associated with a 46% increase in the risk of clinical or histologic progression (adjusted hazard ratio [AHR], 1.46; 95% confidence interval [CI], 1.13-1.87; P for the trend = .004). Compared with patients in the lowest quartile of cholesterol intake (32-152 mg/day), those in the 3rd (224-310 mg/day; AHR, 2.83; 95% CI, 1.45-5.51) and 4th quartiles (>310 mg/day; AHR, 2.74; 95% CI, 1.22-6.16) had significantly increased risk of disease progression.
Notice there is no real difference between the 3rd and 4th quartiles (the second quartile isn't mentioned, but the incremental figure of AHR 1.46 is close enough to what it must be). People who ate the most cholesterol (4th quartile) actually had the same risk of cirrhosis as people who ate a lower amount (3rd quartile). There was a limit to the increased risk. Normally a broken dose-response relationship like this would ring alarm bells, but we are talking about cholesterol, and if dietary cholesterol intake is high enough, the liver's production is inhibited. So it seems to me plausible that the effect is more or less plateaued at high intakes, and this implies that endogenous cholesterol production is also implicated.

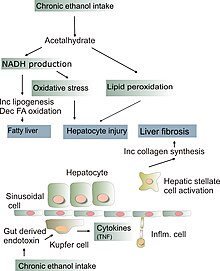
A diet high in polyunsaturated fats increases cholesterol production by the liver (because it lowers serum cholesterol), and a high-carbohydrate diet (esp sugar, or excess starch) also increases the liver's cholesterol production (because it increases triglycerides which need to be packaged with cholesterol). If the liver is fatty, not only the fat but also the extra cholesterol will be retained in the liver. If there is also cholesterol in the diet, this increases the load, and eventually makes inner mitochondrial membranes (cristae) deformed (cristae need to expand and contract in response to energy requirements; the consequent rearrangement of the various enzyme complexes regulates their activity; cholesterol is a stiffening agent that regulates the fluidity and integrity of membranes), so the cholesterol-laden mitochondria find it hard to produce energy or clear substrates, make more free radicals, and precipitate fibrosis.

(This image shows how the accumulation of unesterified cholesterol deforms the membranes of red blood cells in patients with liver disease. Cholesterol is mostly esterfied by taurine and glycine, which are protective against fibrosis).
This is what I think happens. It could happen without dietary cholesterol, is two and a half times more likely to happen with high intakes of it - but depends on a diet that produces fatty liver, plus in this case a virus that promotes similar changes - the dietary cholesterol then being the last straw. In the context of a diet and lifestyle that is protective against fatty liver, dietary cholesterol ought not to be an issue.
Lowering free cholesterol by esterification with taurine is a rational treatment for NASH:
Dig Dis Sci. 2006 Dec;51(12):2225-34. Epub 2006 Nov 2.
The restorative effect of taurine on experimental nonalcoholic steatohepatitis.
Source
Department of Gastroenterology, Changzheng Hospital, Second Military Medical University, Shanghai, 200003, China.
Abstract
Our objective was to explore the restorative effect of taurine on experimental nonalcoholic steatohepatitis (NASH). Thirty-six SD rats were randomly divided into three groups, 12 in each group: the normal group was fed standard rat diet; the model group and the treatment group were both fed a high-fat rat diet for 12 weeks, and the rats in the treatment group were simultaneously injected with taurine subcutaneously for 8 weeks. Hepatic histological change was observed; TNF-alpha and TGF-beta(1) protein expression was identified by immunohistochemistry; mRNA expression of TNF-alpha, TGF-beta(1), type I procollagen, and adiponectin was measured by RT-PCR; body weight, weight gain, liver weight, and liver index were measured; and biochemical parameters monitored included serum transaminases, serum lipids, fasting plasma glucose, and hepatic level of oxidative stress. Rats in the model group showed a significant increase in liver weight, liver index, serum transaminase activities, serum triglyceride, fasting plasma glucose, and oxidative stress; the mRNA expression of TNF-alpha, TGF-beta(1), and type I procollagen increased, whereas the expression of adiponectin decreased significantly, compared with that in the normal group. The typical hepatic lesions of NASH were observed histologically in the model group. Taurine treatment resulted in a significant decrease in liver weight, liver index, serum transaminase activities, serum triglyceride, fasting plasma glucose, and oxidative stress; the mRNA expression of TNF-alpha, TGF-beta(1), and type I procollagen decreased, but the expression of adiponectin increased significantly, compared with that in the model group. Histological improvement was observed in the treatment group. In conclusion, taurine could inhibit lipid peroxidation, improve lipid and glucose metabolism, decrease synthesis of TNF-alpha and TGF-beta(1), promote synthesis of adiponectin, and have a restorative effect on experimental NASH.